CARBON FIBERS INDUSTRY
Carbon fibers or carbon fibres (alternatively CF, graphite fiber or graphite fibre) are fibers about 5–10 micrometres in diameter and composed mostly of carbon atoms.
To produce a carbon fiber, the carbon atoms are bonded together in crystals that are more or less aligned parallel to the long axis of the fiber as the crystal alignment gives the fiber high strength-to-volume ratio (making it strong for its size). Several thousand carbon fibers are bundled together to form a tow, which may be used by itself or woven into a fabric.
The properties of carbon fibers, such as high stiffness, high tensile strength, low weight, high chemical resistance, high temperature tolerance and low thermal expansion, make them very popular in aerospace, civil engineering, military, and motorsports, along with other competition sports. However, they are relatively expensive when compared with similar fibers, such as glass fibers or plastic fibers.
Carbon fibers are usually combined with other materials to form a composite. When combined with a plastic resin and wound or molded it forms carbon-fiber-reinforced polymer (often referred to as carbon fiber) which has a very high strength-to-weight ratio, and is extremely rigid although somewhat brittle. However, carbon fibers are also composited with other materials, such as with graphite to form carbon-carbon composites, which have a very high heat tolerance.
Composite materials
Carbon fiber is most notably used to reinforce composite materials, particularly the class of materials known as carbon fiber or graphite reinforced polymers. Non-polymer materials can also be used as the matrix for carbon fibers. Due to the formation of metal carbides and corrosion considerations, carbon has seen limited success in metal matrix composite applications. Reinforced carbon-carbon (RCC) consists of carbon fiber-reinforced graphite, and is used structurally in high-temperature applications. The fiber also finds use in filtration of high-temperature gases, as an electrode with high surface area and impeccable corrosion resistance, and as an anti-static component. Molding a thin layer of carbon fibers significantly improves fire resistance of polymers or thermoset composites because a dense, compact layer of carbon fibers efficiently reflects heat.
The increasing use of carbon fiber composites is displacing aluminum from aerospace applications in favor of other metals because of galvanic corrosion issues.
Textiles
Precursors for carbon fibers are polyacrylonitrile (PAN), rayon and pitch. Carbon fiber filament yarns are used in several processing techniques: the direct uses are for prepregging, filament winding, pultrusion, weaving, braiding, etc. Carbon fiber yarn is rated by the linear density (weight per unit length, i.e. 1 g/1000 m = 1 tex) or by number of filaments per yarn count, in thousands. For example, 200 tex for 3,000 filaments of carbon fiber is three times as strong as 1,000 carbon filament yarn, but is also three times as heavy. This thread can then be used to weave a carbon fiber filament fabric or cloth. The appearance of this fabric generally depends on the linear density of the yarn and the weave chosen. Some commonly used types of weave are twill, satin and plain. Carbon filament yarns can be also knitted or braided
Microelectrodes
Carbon fibers are used for fabrication of carbon-fiber microelectrodes. In this application typically a single carbon fiber with diameter of 5–7 μm is sealed in a glass capillary.[11] At the tip the capillary is either sealed with epoxy and polished to make carbon-fiber disk microelectrode or the fiber is cut to a length of 75–150 μm to make carbon-fiber cylinder electrode. Carbon-fiber microelectrodes are used either in amperometry or fast-scan cyclic voltammetry for detection of biochemical signaling.
Catalysis
PAN-based nanofibers can efficiently catalyze the first step in the making of synthetic gasoline (not to be confused with syngas) and other energy-rich products out of carbon dioxide. The process uses a “co-catalyst” system in three steps: (1) EMIM–CO2 complex formation; (2) adsorption of EMIM–CO2 complex on reduced carbon atoms and (3) carbon monoxide formation.
The first step uses an ionic liquid, while graphitic carbon structures doped with other reactive atoms replaced silver to produce the final output. The carbon nanofiber catalyst exhibited negligible overpotential (0.17 V) for carbon dioxide reduction and more than an order of magnitude higher current density compared with silver under similar experimental conditions. The reduction derived from the reduced carbons rather than to electronegative nitrogen dopants. The performance came from the nanofibrillar structure and high binding energy of key intermediates to the carbon nanofiber surfaces.
Flexible Heating
Known for its conductivity, carbon fibers can carry very low currents on their own. When woven into larger fabrics, they can be used to reliably deliver infrared heating in applications requiring flexible heating elements and can easily sustain temperatures past 100 °C due to its physical properties. Many examples of this type of application can be seen in 'DIY' or Do it Yourself heated articles of clothing and blankets. Due to its chemical inertness, it can be used relatively safely amongst most fabrics and materials; however, shorts by the material folding back on itself will lead to increased heat production which can lead to fire.
Each carbon filament is produced from a polymer such as polyacrylonitrile (PAN), rayon, or petroleum pitch, known as a precursor. For synthetic polymers such as PAN or rayon, the precursor is first spun into filament yarns, using chemical and mechanical processes to initially align the polymer atoms in a way to enhance the final physical properties of the completed carbon fiber. Precursor compositions and mechanical processes used during spinning filament yarns may vary among manufacturers. After drawing or spinning, the polymer filament yarns are then heated to drive off non-carbon atoms (carbonization), producing the final carbon fiber. The carbon fibers filament yarns may be further treated to improve handling qualities, then wound on to bobbins.
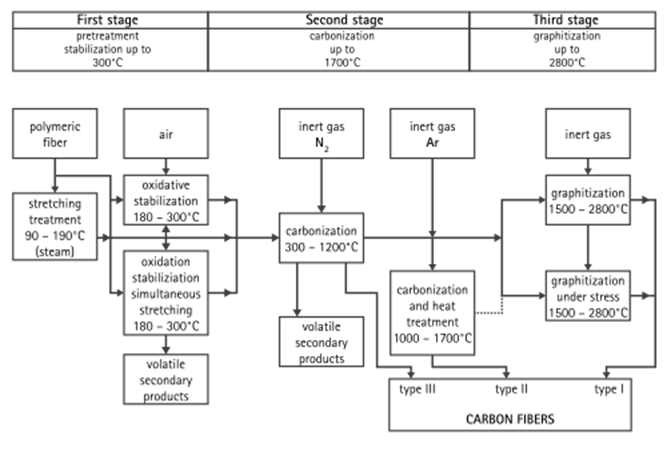
A common method of manufacture involves heating the spun PAN filaments to approximately 300 °C in air, which breaks many of the hydrogen bonds and oxidizes the material. The oxidized PAN is then placed into a furnace having an inert atmosphere of a gas such as argon, and heated to approximately 2000 °C, which induces graphitization of the material, changing the molecular bond structure. When heated in the correct conditions, these chains bond side-to-side (ladder polymers), forming narrow graphene sheets which eventually merge to form a single, columnar filament. The result is usually 93–95% carbon. Lower-quality fiber can be manufactured using pitch or rayon as the precursor instead of PAN. The carbon can become further enhanced, as high modulus, or high strength carbon, by heat treatment processes. Carbon heated in the range of 1500–2000 °C (carbonization) exhibits the highest tensile strength (820,000 psi, 5,650 MPa or N/mm²), while carbon fiber heated from 2500 to 3000 °C (graphitizing) exhibits a higher modulus of elasticity (77,000,000 psi or 531 GPa or 531 kN/mm²).
Renewable fiber production research
Currently a number of research institutions are carrying out research to try to synthesise carbon fiber from renewable, non-fossil fuel based feedstocks. This could reduce greenhouse gas emissions associated with carbon fiber manufacture as well as long term costs of production.
PRODUCTIONS SCHEME

Graddo Invest Management, s.r.o.
EU, Czech Republic, Rumy 1596, 760 01 Zlín
U EU, Czech Republic, Bulhara 3, 110 00 Prague
Phone: +420 577 210 994
Fax: +420 577 217 541